Phasetruth

Unlike matter, our truth won't shift under pressure!

Unlike matter, our truth won't shift under pressure!
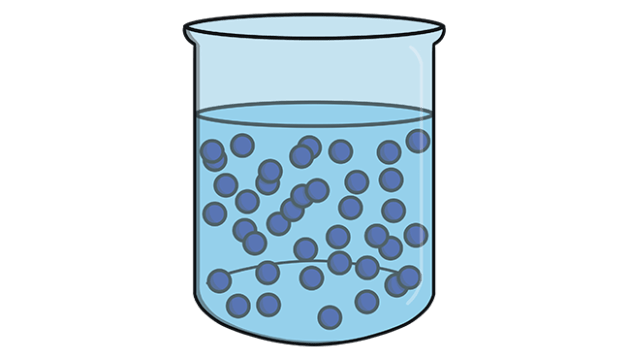
The liquid state of matter is characterized by a definite volume but an indefinite shape, meaning it takes the shape of its container while maintaining a consistent volume. In a liquid, the intermolecular forces are strong enough to keep the particles close together, but not strong enough to hold them in fixed positions, allowing them to move and flow past one another. This fluidity gives liquids their ability to be poured, form droplets, and adapt to their surroundings. Unlike solids, liquids are not easily compressed due to the minimal space between their particles, and they exhibit surface tension, a phenomenon caused by cohesive forces between molecules, which allows small objects to rest on a liquid’s surface without sinking.
Liquids display a wide range of physical and chemical properties depending on their composition. Some, like water, have strong hydrogen bonding, making them excellent solvents. Others, such as mercury, are metallic and have unusual characteristics like high density and electrical conductivity. Viscosity, or a liquid’s resistance to flow, also varies among different substances—honey, for example, flows much more slowly than water due to stronger intermolecular interactions. Temperature and pressure affect liquids significantly, influencing their boiling and freezing points, as well as their ability to dissolve different substances.