Phase change refers to the transformation of a substance from one state of matter to another, driven by changes in energy, particularly temperature and pressure. These transformations occur at the molecular level, where particles gain or lose energy, causing them to either move closer together or spread farther apart. The classic phase changes include melting, freezing, evaporation, condensation, sublimation, and deposition, each describing the transition between specific phases.
Beyond these common changes, there's also the transition from gas to plasma and plasma to gas. Ionization happens when a gas gains enough energy (often through heat or electrical input) to strip electrons from its atoms, creating a plasma, a highly energized state of matter where electrons and ions move freely. This process can be seen in things like neon signs or stars. Recombination, the reverse, happens when plasma cools, and the free electrons combine with ions to form neutral atoms, turning the plasma back into a gas, as seen when lightning dissipates.
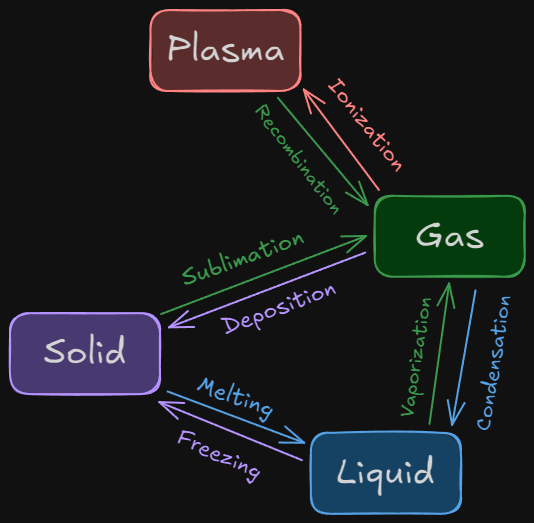
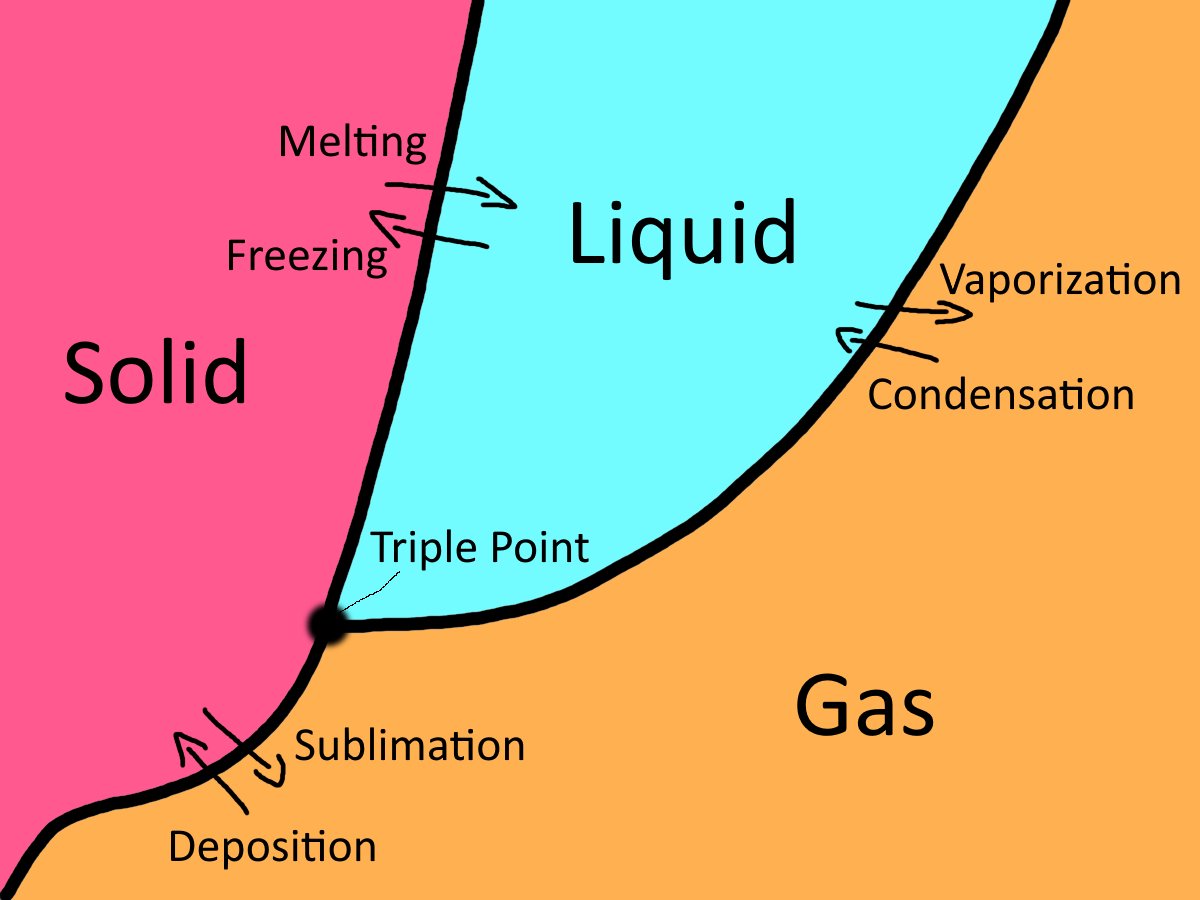
- Melting occurs when a solid gains enough heat to break free from its rigid structure and becomes a liquid. When ice melts, its water molecules, which were once tightly bound, begin to move freely, turning the solid into liquid water.
- Freezing, the reverse of melting, happens when a liquid loses heat, causing its molecules to slow down and come together, forming a solid—like water freezing into ice.
- Evaporation is the process by which a liquid turns into a gas as the molecules at the surface gain enough energy to break free of the liquid state, like when water in a puddle evaporates into steam. Condensation is the opposite, where a gas loses energy, cooling and becoming a liquid again, like dew forming on a cold morning.
- Condensation is the process where a gas loses heat and changes back into a liquid. This happens when gas molecules slow down and come together, forming liquid droplets. A common example is when warm, moist air hits a cold surface, like when water vapor condenses on a bathroom mirror after a hot shower.
- Sublimation occurs when a solid skips the liquid phase entirely and transforms directly into a gas, such as when dry ice (solid carbon dioxide) turns into carbon dioxide gas without first becoming liquid. Deposition is the reverse process, where a gas transitions directly into a solid, as seen when frost forms on a surface in cold weather.
- Deposition is the opposite of sublimation, where a gas transitions directly into a solid without passing through the liquid phase. This occurs when gas molecules lose enough energy that they arrange themselves into a solid structure. A great example of deposition is the formation of frost on a cold window—water vapor in the air directly changes into solid ice crystals without ever becoming liquid.



